Entrapped paclitaxel
Cytotoxic cargo sequestred in the particle's porous network
Pharma in silica engineers a game-changer for millions of cancer patients: a treatment that improves outcomes and reduces harm
Current chemo often brings limited improvements in survival and remission, in part because it disrupts the immune system, inhibiting the patient’s natural ability to neutralize cancer cells. Chemotherapy also imposes painful, long-lasting, and costly side effects (anemia, infections, neurological disorders, discomfort, etc.).
Cytotoxic drugs are nevertheless routinely used alongside surgery, radiation, immunotherapy, hormone therapy, and cell-based treatments, generating over $17 billion in annual sales. Shockingly chemotherapy remains one of the most under-studied treatments in oncology: few clinical trials focus on the safety and effectiveness of these treatments, exposing patients to regressive therapeutic methods.


Cytotoxic cargo sequestred in the particle's porous network
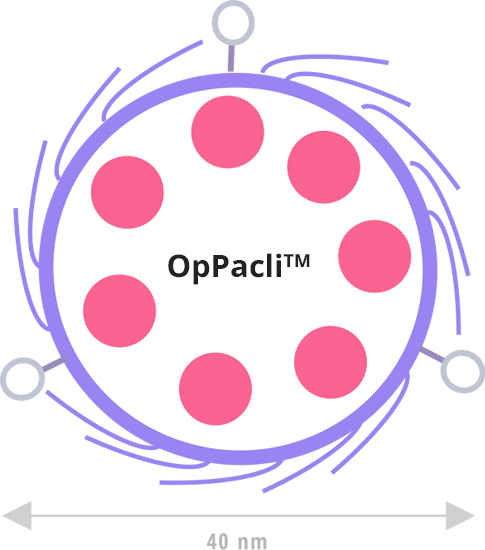
Proprietary combination of silanes (regulator-accepted excipient)
Surface treatment for solubility, stability, stealth in blood, etc.
Anchoring points for third-party targeting ligands & other useful functions
Injection in saline (no-toxic solvent)
No early drug release during extensive circulation in the blood stream, minimizing off-target effects
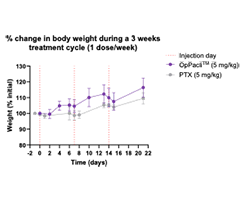
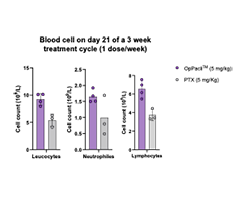
Rapid recovery of weight & blood cells & absence of adverse effects such as anemia, neutropenia, thrombocytopenia
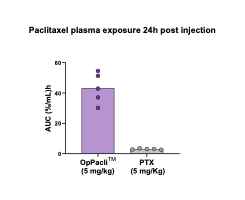
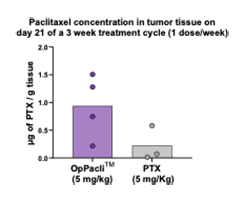
Preferred delivery: 5x more paclitaxel to the tumor compared to standard of care (Taxol®, Abraxane®)
Drug release triggered by the tumor environment
Pharma in silica‘s intends to leverage its mastery of silica chemistry and early-stage product development to partner with pharmas, like the swiss Debiopharm does with organic chemistry & the Vancouver-based abCellera with bivalent antibodies. The first partnerships, licenses at technical and human proof-of-concept stages, will eventually become longer-term co-development endeavors.

Paclitaxel sequestred in 40 nm silica-made biocompatible nanoparticles
Surfactant-free, patented process
Own production capacity

CMC and study plans compliant with pre-IND/pre-CTA meetings. Toward a North-American phase Ib/IIa trial in advanced cancer patients
42 patients, aiming for superior safety & efficacy
| ImPacli™ | Metronomic paclitaxel to boost immune response in solid cancers |
| OpCombo™ | Multiple-compound with synchronized release for cancer or hepatic indications |
| OpKinib™ | In-licensed novel kinase inhibitor with controlled-release in solid cancers |

The Pharma in silica team believes a true precision chemotherapy will emerge from diverse additions to the OpPacli™ nanocarrier.
Any ideas?
Targeting, cellular binding, internalization & trafficking strategies
Alternatives to PEG for solubility & stealth in blood
Class IV BSC and other difficult APIs (hydrophobic small molecules)
Efficacy enhancers
Administration methods & devices
Solvent-free solubilization methods
Understanding of pharmacokinetics of nanoparticles, mechanistic of tumors & cancer biology
Any other audacious (including crazy) & useful idea
We look forward to work with you via industrial codevelopment, grant-supported collaborative research, licensing or acquisition of promising solutions.
Pharma in silica launched an international call for solutions at the summer of 2025.
Our core expertise consists of the chemistry of silica, early-stage development of nanoproducts & dealmaking. But fighting cancer is a team sport.
Complementary knowledge, technologies & components can contribute to a universal, safe, efficient & affordable chemotherapy.
Three areas of pharmas’ catalogs can be uniquely well served by our silica nanocarrier. We call them toxic hits, nearly successful & second chances (see below).
For each area, compatible partners will be identified via interviews with KOLs and review of publications, posters, patents, regulatory filings, company websites, clinical trial registers, stock market and sales reports, press releases, etc.
Asset-specific value propositions, market study and deal structure will be prepared for the top prospects in each area.
Pharmas will be approached via an internal champion (responsible researcher or executive) rather than the pharma’s gate keepers.
Licensing & preclinical & clinical lot supply agreements will be first sought, and, later-on, co-development agreements.
Chemotherapy blockbusters with suboptimal therapeutic profile
Clinically-validated cytotoxic drugs enhanced by Pharma in silica's technology (OpPacli™ for solid tumors; other cytotoxic drugs for solid cancers)
Development-stage novel or failed oncology assets
Pharma's proprietary assets with high therapeutic potential but constrained by toxicity, formulation, bio availability, bad PK, etc.
Under-performing onco-drugs
Commercialized oncology drugs candidate for improvement, patent extension, additional indications and/or relaunch.

Co-founder of Medicago (Quebec), CEO of ERA Biotech (Barcelona)

Nanomaterial science for biomedical applications & imaging at Université Laval (Québec)

PK/PD & nanomaterial science for biomedical applications at Université Laval (Québec)

Oncology R&D/business dev. & tech transfer

Oncologist expert in chemo & early-stage drug development

Regulatory, product dev., CMC & process dev., intellectual property, tax credits, reimbursements, law & accounting
Canadian private corporation (provincial charter)
Shareholders: accredited investors, Investment Québec,
cofounders (no controlling shareholder)
Mr François Arcand MBA, President
arcandf@pharma-insilica.com
2500 boul. du Parc-Technologies, Quebec, Qc Canada G1P 4S6
The company is determined to achieve four worthy milestones via an 18-month, 6 M USD program. The allocation of a 4 M USD seed round is recited in the adjoint table.
Additional non-dilutive funds will be provided by advances on SR&RD credits (± 1,5 M USD) and credit financing for CMC-related CAPEX expenditures (± 250 k USD). Grants and exploratory payments by pharma partners are probable, will be sought and have not been budgeted.
Pharma in silica’s shareholders have so far committed 500 k USD to this subscription. Québec-based institutional funds, early-stage VCs & business angel groups are likely to follow the lead of an expert investor.
Pharma in silica raised 4,5M CAD in seed capital since 2019 (latest subscription: 1,5 M CAD, pre-money value of 12 M CAD, August 2024).
A clinical round of ± 6 M USD is planned by the end of 2026 to finance:
| Preclinical study, OpPacli™ | Bring the first product candidate to patients with late-stage NSCLC in a phase Ib/IIa | 1,75 M |
| Strategic endeavors | Elevate production capacity from preclinical to FDA-compliant clinical grade and scale; patent maintenance & filing | 1 M |
| Precision chemo platform | Identify and integrate complementary technologies via own-& collaborative research, licensing & acquisition | 1 M |
| Business development | Prepare tailored proposals for partnerships with pharmas | 250 K |
Corporate, technical & financial information are available upon request. Laboratories Pharma in silica inc. is audited as per the international financial reporting standards.
